SAN CLEMENTE, CA / ACCESSWIRE / July 1, 2021 / ReShape Lifesciences Inc. (NASDAQ:RSLS), the premier physician-led weight-loss solutions company, today announced that Lap-Band® utilization has increased since COVID-19 in response to patients' increased desire for a reimbursed, effective, and sustainable weight-loss procedure that can be provided in outpatient surgery centers. The Lap-Band is clinically proven to be the safest bariatric procedure available on the market, backed by over 20 years of data demonstrating lower complication and mortality rates compared to other surgical weight-loss procedures. The Lap-Band is also the only FDA-approved, laparoscopic weight-loss device specifically indicated for lower Body Mass Indexes of 30 or higher in the U.S. Following this procedural growth and the favorable evaluation of recent and historical consumer focused marketing efforts, with Lap-Band annual revenue of approximately $300M at one time, the company intends to significantly enhance and broaden patient-direct advertising and promotion.
The pandemic has served as an eye-opener to the obesity epidemic, with over seventy percent of American adults now overweight, and more than a third are obese. A recent Harris poll reported that a majority of adults (61%) experienced undesired weight changes since the pandemic started, with 42% reporting they gained more weight than they intended (gaining an average of 29 pounds). Numerous health issues have been associated with obesity for many years, with obese individuals more likely to develop a number of serious health conditions, including Type 2 Diabetes, heart disease and certain cancers. Recently, obesity has been identified as a leading risk factor for death among those critically ill with COVID-19.
"The attractiveness of Lap-Band surgery to patients is that the complication rate is markedly lower compared to other weight-loss procedures," said Christine Ren-Fielding, MD, FACS, Professor of Surgery, NYU School of Medicine, Division of Bariatric Surgery, Director, NYU Langone Weight Management Center. "Lap-Band is a valid first-line intervention for a lifelong chronic condition because it is safe, effective and reversible. The Lap-Band is also unique in that it can be adjusted, making it an interactive device through life conditions that can include a woman's course of pregnancy."
ReShape Lifesciences recognizes that a number of Lap-Band practices have increased their procedures year over year, and more relevantly, when comparing the time period of January - May 2021 to the same time period in 2019 (pre-COVID-19). The company's first reimbursed telehealth service for weight loss, reshapecare™, was also launched as a physician-prescribed therapy. The reshapecare program helps physician practices reduce administrative burden and increases engagement with patients to improve their aftercare and outcomes.
"Over the past three years, the Lap-Band of Louisville Program has seen steady volume growth of patients with a lower BMI," said John Olsfoka, MD, University of Louisville Health, Mary & Elizabeth Hospital Bariatric Program, Lap-Band of Louisville."Research has shown that patients who undergo weight-loss surgery when their BMI is less than 40 are more likely to achieve improvement of obesity-related conditions. It is our goal to give patients a tool that is adjustable, adaptable and safe with sustainable weight loss."
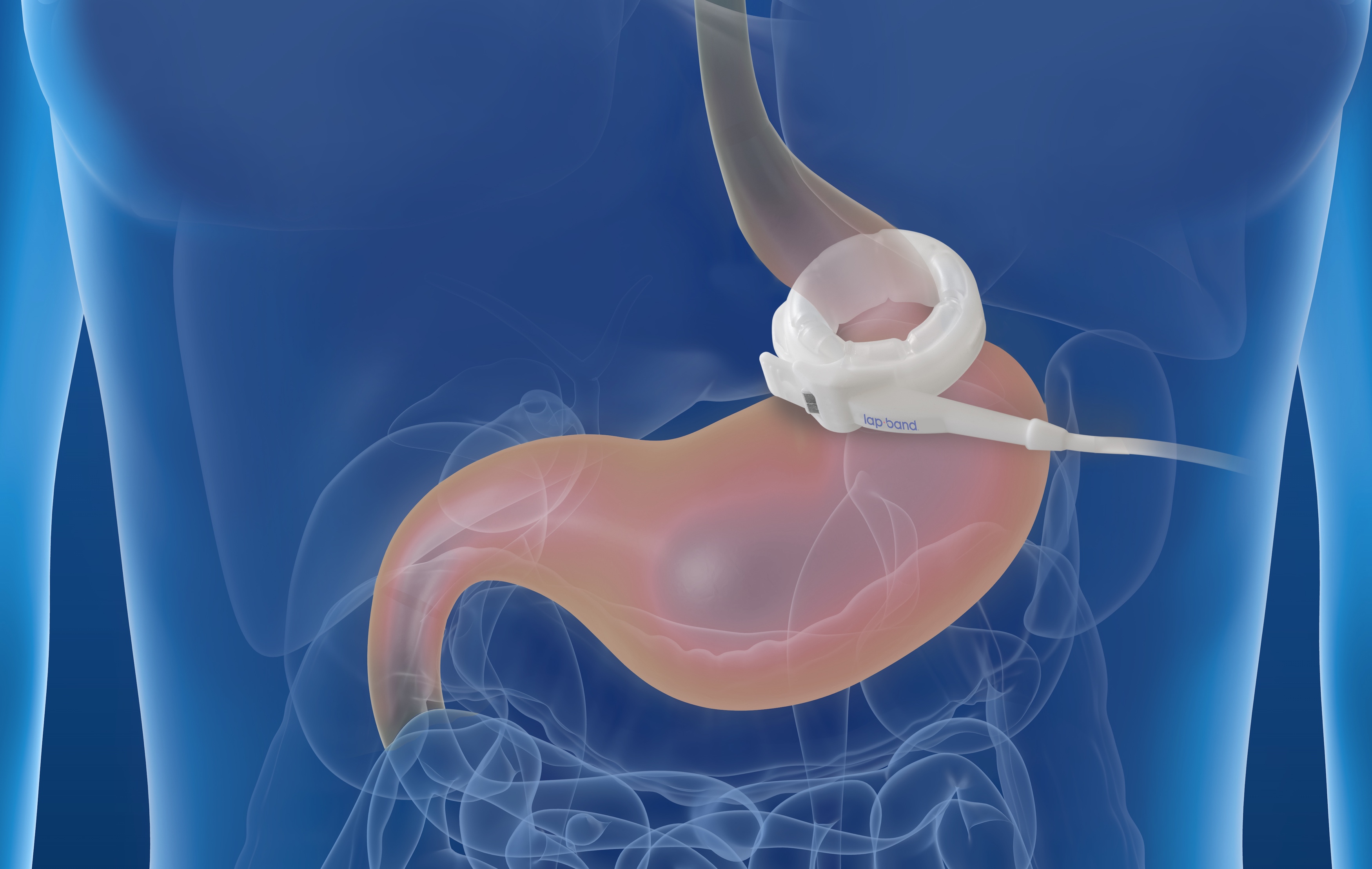
The Lap-Band procedure has been performed worldwide over 1,000,000 times since 1993 and is reimbursed for eligible patients by most insurance companies. The Lap-Band does not permanently alter anatomy, and offers a reduced risk of vitamin and mineral deficiencies as food is absorbed using a person's natural, unaltered digestive tract.
ABOUT RESHAPE LIFESCIENCES INC.
ReShape Lifesciences™ is America's premier weight-loss and metabolic health-solutions company, offering an integrated portfolio of proven products and services that manage and treat obesity and metabolic disease. The FDA-approved Lap-Band® Program provides minimally invasive, long-term treatment of obesity and is an alternative to more invasive surgical stapling procedures such as the gastric bypass or sleeve gastrectomy. The ReShape Vest™ System is an investigational (outside the U.S.) minimally invasive, laparoscopically implanted medical device that wraps around the stomach, emulating the gastric volume reduction effect of conventional weight-loss surgery. It helps enable rapid weight loss in obese and morbidly obese patients without permanently changing patient anatomy. reshapecare™ is a virtual weight-management program that supports lifestyle changes for all weight-loss patients led by board certified health coaches to help them keep the weight off over time. The recently launched ReShape Marketplace™ is an online collection of quality wellness products curated for all consumers to help them achieve their health goals.
Forward-Looking Safe Harbor Statement:
This press release may contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements generally can be identified by the use of words such as "expect," "plan," "anticipate," "could," "may," "intend," "will," "continue," "future," other words of similar meaning and the use of future dates. These forward-looking statements are based on the current expectations of our management and involve known and unknown risks and uncertainties that may cause our actual results, performance, or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. These risks and uncertainties are described more fully in the Company's filings with the Securities and Exchange Commission, particularly those factors identified as "risk factors" in our annual report on Form 10-K filed March 11, 2021. We are providing this information as of the date of this press release and do not undertake any obligation to update any forward-looking statements contained in this document as a result of new information, future events or otherwise, except as required by law. The information included on any websites referred to in this press release is not incorporated by reference into this press release and investors should not rely on any such information in deciding whether to invest in our securities.
ReShape Lifesciences Investor Contact:
Thomas Stankovich
Chief Financial Officer
949-276-6042
[email protected]
Investor Contacts:
James Salierno/Daniel Kontoh-Boateng
Vice President
The Ruth Group
646-536-7028/7019
[email protected]
[email protected]
SOURCE: ReShape Lifesciences Inc.




