GOLDEN, CO / ACCESSWIRE / June 26, 2018 / Vitro Diagnostics, Inc. (OTCQB: VODG), dba Vitro BioPharma, announced its 2nd quarter ended April 30th 2018 financial results of operations.
Vitro Diagnostics Inc. (''Vitro'') is pleased to announce a record 2nd quarter in Stem Cell Revenues. Vitro recorded 2nd quarter revenues of $125,886 vs $56,633 an increase of 222% over the same comparative quarter last year. In addition, Stem Cell therapies accounted for 53% of the revenues up from only 8% of the revenues in the prior comparative quarter last year. Current quarter stem cell revenues from our advanced stem cell therapies with our offshore partners was $66,537 for the 2nd quarter ended April 30th, 2018 vs $4,594 for the 2nd quarter ended April 30th, 2017.
The company's gross profit margins improved to 75% up from 50% in the comparative prior year's quarter. Gross margin improvement is in line with the strategic direction of the company to expand the market in its offshore Stem Cell therapies. The company's clean-room lab expansion last year and increase in its batch Stem Cell manufacturing and production capacity to 2 Billion cells per harvest, using its patent-pending cell line, has increased efficiencies and lowered the cost per harvest.
Overall operating expenses increased in the quarter to $121,080 from $106,068 in the prior year's comparative quarter. The increase in expenses reflects the addition of additional team resources as the Company expands its capability to service its strategic direction of offshore Stem Cell sales. The company has added internal operations staff in the stem cell lab; outside consultants supporting its efforts towards CLIA and ISO certification as well as the addition of internal accounting, finance and administrative support staff compared to the prior year.
The company raised financing and reduced debts for its expansion from the issuance of 3 year convertible notes; due December 31st 2021, bearing interest at 10% per annum; convertible into the common stock of the company at $0.05 cents per share. During the quarter $137,500 of the notes were converted to 2,700,000 shares of common stock. As at April 30th, 2018 there were $238,750 of outstanding notes and deferred interest. Subsequent to April 30th the company has issued an additional $285,000 of notes from fund raising activities.
The company's CEO converted $1,003,119 of his debts to 20,062,378 shares of common stock. As at April 30th the company's founder, C.E.O. and Chairman of the Board owned approximately 57% of the company's common stock. The company entered into 5 year employment agreements with its C.E.O., C.O.O. and C.F.O. establishing an experienced executive team committed to the company's aggressive growth plans
During the quarter the company achieved and pursed the following company objectives;
- Accelerated the Company's Marketing Program
We had previously worked with a New Zealand patient diagnosed with a type of Parkinson's disease called Multiple System Atrophy (MSA), a fatal condition without effective treatment. She had previously received our stem cell activation therapy, NutraVivo™ that helped to stabilize her but without changing her symptoms. She has now received 3 transplants of stem cells manufactured by Vitro Biopharma and had subsequent reversal of some neurological symptoms.
- This trial has been expanded to include 6 patients with neurodegenerative diseases, including MSA and ALS.
- Regulatory approval was obtained through a New Zealand statute comparable to the ''Right to Try'' in the US.
- Preliminary results continue to show clinical safety of the procedure while showing reversals of some neurological symptoms.
- During the quarter our Cayman Island partner launched its new medical tourism web site and added a marketing manager. www.dvcstem.com
- Expanded opportunities for stem cell therapies in the US:
We continued the initial commercialization of NutraVivo™, our patent-pending nutraceutical formulation for treatment of Concussion/TBI by establishing initial testing and publication of evidence for stem cell activation.
https://vitrobiopharma.com/stem-cell-activation-by-nutravivo-dietary-supplements/
- Began Social Media postings
https://www.instagram.com/vitro.biopharma/
- Hired an IT firm for complete integration of marketing, social media and web presence.
- Research and Development:
- Developing methods for topical stem cell delivery and enhanced penetration of the blood-brain barrier.
- Completed comparative study of MSCs derived from adipose, placental, bone marrow and umbilical cord tissue: Results show bio-similarity with superior potency & differentiation capacity of our patent-pending MSCs derived from umbilical cord.
Dr. Jim Musick, CEO/CSO said, ''We are pleased to see continued revenue growth due to the expansion of our prior R&D operations to include stem cell therapies initially targeting off-shore clinics while pursing US markets as well. Our existing operations in Australia, Grand Cayman Islands and Eastern Europe continue to expand. During our second quarter, we added supply of stem cell therapies to a clinical partner in New Zealand. Regulatory authority was obtained through a statutory provision like the ''right to try'' in the US that was recently approved by the US federal government. The initial results are promising, and our protocol includes longer term follow-up with more quantitative diagnostics to compare pre-and post-treatment results. We plan to continue these studies and expand to broader clinical trials of neurological disease & conditions. Operations in the US are initially focused on stem cell activation therapies for concussion/TBI using our patent-pending NutraVivo™ nutraceutical formulation that has been shown to activate neural stem cells. Thus, our overall strategy provides numerous revenue streams from off-shore operations while developing stem therapies targeting select US markets positioning the Company for substantial revenue growth and increased shareholder value.''
In summary, Vitro Biopharma is advancing as a key player in regenerative medicine with 10 years' experience in the development and commercialization of stem cell cell products for research, recognized by a Best in Practice Technology Innovation Leadership award for Stem Cell Tools and Technology and a growing track record of successful translation to therapy. We plan to leverage our proprietary technology platform to the establishment of international Stem Cell Centers of Excellence and regulatory approvals in the US.
Sincerely yours,
James R. Musick, PhD.
CEO & CSO
www.vitrobiopharma.com
Forward-Looking Statement
Statements herein regarding financial performance have not yet been reported to the SEC nor reviewed by the Company's auditors. Certain statements contained herein and subsequent statements made by and on behalf of the Company, whether oral or written may contain ''forward-looking statements''. Such forward looking statements are identified by words such as ''intends,'' ''anticipates,'' ''believes,'' ''expects'' and ''hopes'' and include, without limitation, statements regarding the Company's plan of business operations, product research and development activities, potential contractual arrangements, receipt of working capital, anticipated revenues and related expenditures. Factors that could cause actual results to differ materially include, among others, acceptability of the Company's products in the market place, general economic conditions, receipt of additional working capital, the overall state of the biotechnology industry and other factors set forth in the Company's filings with the Securities and Exchange Commission. Most of these factors are outside the control of the Company. Investors are cautioned not to put undue reliance on forward-looking statements. Except as otherwise required by applicable securities statutes or regulations, the Company disclaims any intent or obligation to update publicly these forward-looking statements, whether as a result of new information, future events or otherwise.
CONTACT:
Dr. James Musick
Chief Executive Officer
Vitro BioPharma
(303) 999-2130 Ext. 3
E-mail: [email protected]
www.vitrobiopharma.com
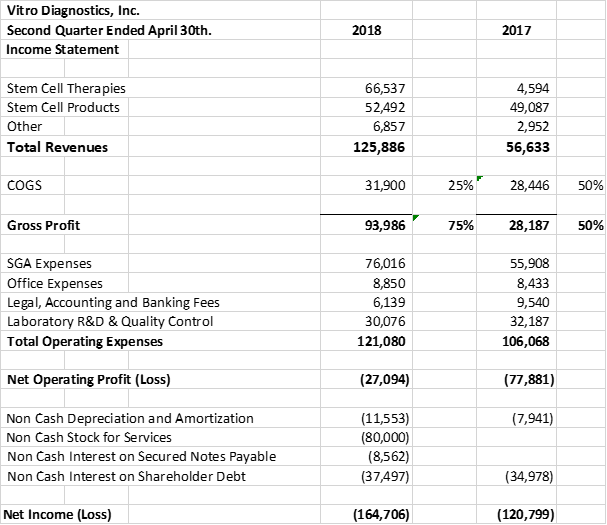
The company provides its financial information for investor purposes only, the results published are not audited or necessarily SEC or GAAP compliant.
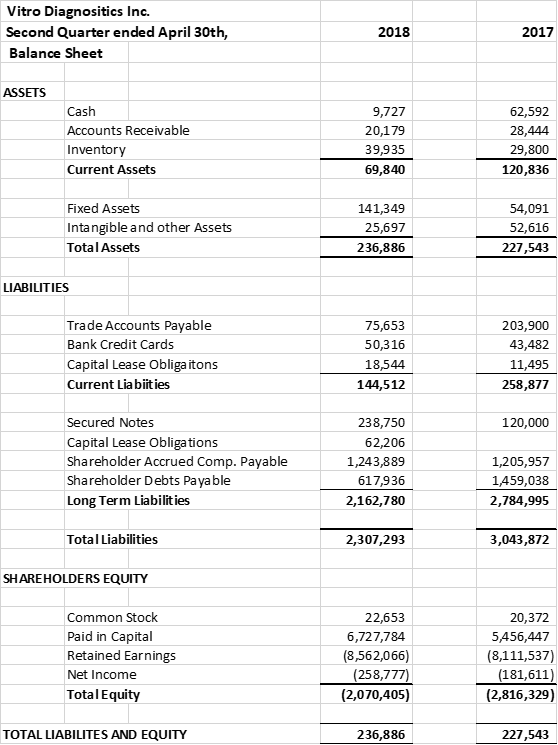
The company provides its financial information for investor purposes only, the results published are not audited or necessarily SEC or GAAP compliant.
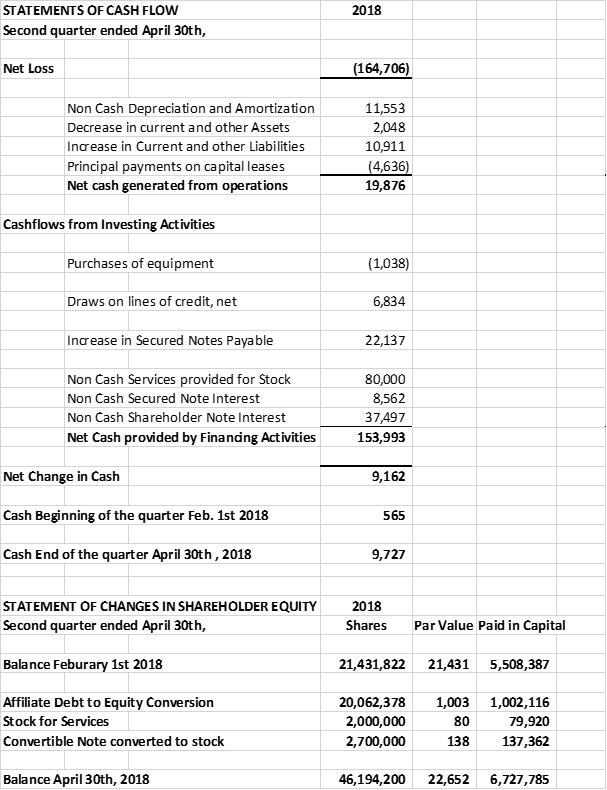
The company provides its financial information for investor purposes only, the results published are not audited or necessarily SEC or GAAP compliant.
SOURCE: Vitro Diagnostics, Inc.




