DehydraTECHTM-powered semaglutide achieved these benefits in a human pilot study compared to Rybelsus®:
- Sustained lower levels of blood glucose from baseline including nearly 10x lower after 24 hours;
- Lower blood-glucose spike after eating; and
- Successful first-ever DehydraTECH test with a "large molecule" drug.
KELOWNA, BC / ACCESSWIRE / November 28, 2023 / Lexaria Bioscience Corp. (NASDAQ:LEXX) & (NASDAQ:LEXXW) (the "Company" or "Lexaria"), a global innovator in drug delivery platforms announces additional positive interim results from a human pilot study (the "Study") evaluating DehydraTECHTM technology for the oral delivery of the glucagon-like peptide-1 ("GLP-1") drug semaglutide available commercially in the branded product Rybelsus®, further to its previously announced findings from this work.
The Study was performed by a prominent university research center comparing a single 7 mg semaglutide dose of a Rybelsus tablet ("Control") to a matching dose from Rybelsus that had been compound formulated in capsule form using DehydraTECH processing technology enhancements ("DehydraTECH GLP-1").
Blood Glucose Levels
It is accepted by the Food and Drug Administration ("FDA") that, "one role of GLP-1 is to prompt the body to produce more insulin, which reduces blood glucose (sugar)." Because blood glucose levels are a key consideration in control of diabetes and other health conditions, the Study measured blood glucose levels at each of the 19 sample time points.
Blood Glucose Levels
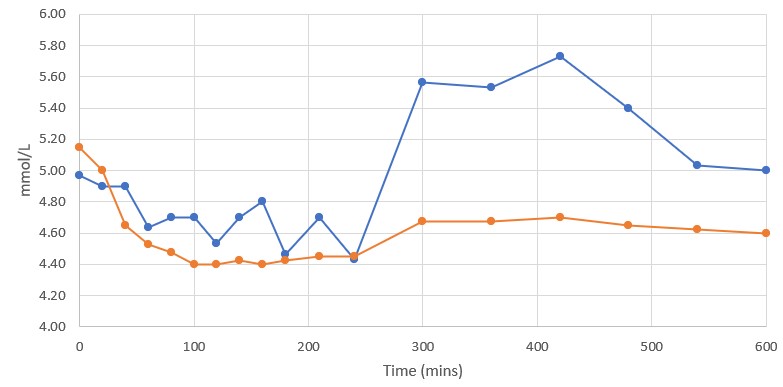
Rybelsus (blue) 7mg (n=3) DehydraTECH (orange) GLP-1 7mg (n=4)
The Control group evidenced reduced blood glucose levels by between 1.3% and 6.7% relative to the time zero baseline during the first 100 minutes of the Study. The DehydraTECH GLP-1 group evidenced reduced blood glucose levels by between 2.9% and 14.6% relative to baseline during those same initial 100 minutes. At all but the 20-minute and 240-minute sample time points, the DehydraTECH GLP-1 blood glucose levels were reduced more than evidenced by the Control group.
Notably, even as long as 24 hours after dose administration, the blood glucose levels were reduced in the DehydraTECH GLP-1 group by 6.3% relative to baseline while the blood glucose level evidenced in the Control group was only reduced by 0.67%, evidencing nearly a ten-fold improvement with the DehydraTECH GLP-1. (Only the first 10 hours of study results displayed in the graph above.)
Of particular note, blood glucose levels spiked by 22.7% in the Control group after the subjects were permitted to eat a standardized meal at the 240-minute mark and a standardized snack at the 360-minute mark. Although the DehydraTECH GLP-1 subjects consumed choices of similar meals and snacks at the same times, their blood glucose levels rose by only 5.3%. It is not presently known whether the higher sustained levels of blood semaglutide delivery by DehydraTECH GLP-1 enabled greater efficacy in achieving sustained blood glucose reduction, thereby helping to attenuate the postprandial spikes in blood glucose experienced in the Control group, although this may be a plausible explanation for the difference.
This Study is only meant to provide early-stage indicative information to Lexaria about the possibility of enhancing the pharmacokinetic ("PK") and pharmacodynamic performance of orally delivered GLP-1 drugs to assist in guiding the Lexaria team in additional investigations. There was minor variability in the diets of the subjects at the 240-minute meal and 360-minute snack intervals noted above during the concentrated 10-hour post dosing monitoring period which could account for some of the differences in the test data, although meal and snack selection allowance was from a set of standardized choices.
Ongoing and Future Work
This Study is not complete and additional results will be reported, likely in late December or early January following the cross-over Study visit as described below. Given the small sample size of this Study, it was not sufficiently powered for statistical significance analysis, which will be a key part of any expanded studies with DehydraTECH GLP-1 undertaken in the future.
Parallel to Lexaria's 2021 optimization program with DehydraTECH-processed cannabidiol ("CBD"), where the formulation utilized in the animal study DIAB-A22-1 demonstrated 364% higher (p=0.0002) PK performance than Lexaria's original DehydraTECH-CBD formulations, we expect to create several different DehydraTECH GLP-1 formulations to explore delivery and performance optimization of semaglutide. As seen with our past DehydraTECH-CBD advancements, Lexaria will endeavor to similarly improve performance of future DehydraTECH-GLP-1 formulations.
About the Study
The Study was performed to provide an early-stage indication of whether DehydraTECH processing could improve oral drug delivery characteristics of the GLP-1 drug semaglutide sold as Rybelsus. A single semaglutide dose of 7 mg of the Rybelsus Control was compared to the matching dose of the DehydraTECH GLP-1, swallowed by each subject after an overnight fasting period together with a 50 mL glass of water. The DehydraTECH GLP-1 formulation used in this Study was compound formulated strictly for research purposes. Seven healthy subjects were dosed, four of whom received the DehydraTECH GLP-1, and three of whom received the Control. These seven subjects are expected to return to the Study site in December to be dosed a second time in the reverse order following the "cross-over" design of this Study to ensure that all seven subjects will have been treated with both the Control and DehydraTECH GLP-1 treatments over the course of the two visits.
Semaglutide is the first so-called "large molecule" ever evaluated with DehydraTECH processing. Lexaria has typically worked with drugs and bioactive substances that are considered "small molecules" with low molecular weights. Large molecules, on the other hand, generally have much larger molecular weights of 3000 daltons or greater, are often but not always biolocially derived and are obviously more complex. At this time Lexaria does not know whether DehydraTECH may offer performance improvements to any significant quantity of large molecule drugs, but, if so, this could represent a radically expanded suite of drugs that may potentially be processed with DehydraTECH.
About Lexaria's Diabetes Study Program.
Lexaria began its DehydraTECH diabetes-related formal studies in 2022. On March 2, 2023 and June 16, 2023 Lexaria announced that in pre-clinical diabetes study DIAB-A22-1 in obese diabetic-conditioned animals, DehydraTECH-CBD achieved each of the following:
- Lowered blood glucose levels by 19.9% (p<0.05)
- Lowered overall body weight by 7% sustained over 8 weeks
- Increased locomotor activity (p<0.05)
- Lowered triglyceride levels by more than 25% (p<0.007)
- Lowered blood urea nitrogen levels by 27.9% (p<0.001)
On August 2, 2023 Lexaria announced its intention to study weight loss and diabetes control in a human population using DehydraTECH-CBD.
DehydraTECH-CBD's ability to reduce blood sugar levels in animals is extremely encouraging and warrants additional investigation. According to the Center for Disease Control, managing your blood sugar levels is important to avoid diabetes-related conditions such as vision loss, heart disease, and kidney disease. Limited research, mostly in animal studies, indicates that without DehydraTECH's noteworthy performance enhancements, generic CBD might be ineffective in controlling blood sugars.
About DehydraTECH
DehydraTECH is a patented drug delivery formulation and processing platform technology Lexaria has developed and is investigating for a variety of beneficial molecules. DehydraTECH is designed to improve the way active molecules enter the bloodstream upon oral ingestion. DehydraTECH has also demonstrated enhanced delivery of certain active molecules into brain tissue, which Lexaria believes to be of particular importance for centrally active compounds. Lexaria has also developed DehydraTECH formulations for other applications demonstrating superior bio-absorption when administered intraorally and topically.
About Lexaria Bioscience Corp.
Lexaria Bioscience Corp.'s patented drug delivery technology, DehydraTECH™, improves the way active pharmaceutical ingredients (APIs) enter the bloodstream through oral delivery. Since 2016, DehydraTECH has repeatedly demonstrated the ability to increase bio-absorption with cannabinoids, antiviral drugs, PDE5 inhibitors and more. DehydraTECH has also evidenced an ability to deliver some drugs more effectively across the blood brain barrier. Lexaria operates a licensed in-house research laboratory and holds a robust intellectual property portfolio with 37 patents granted and many patents pending worldwide. For more information, please visit www.lexariabioscience.com.
CAUTION REGARDING FORWARD-LOOKING STATEMENTS
This press release includes forward-looking statements. Statements as such term is defined under applicable securities laws. These statements may be identified by words such as "anticipate," "if," "believe," "plan," "estimate," "expect," "intend," "may," "could," "should," "will," and other similar expressions. Such forward-looking statements in this press release include, but are not limited to, statements by the Company relating to the Company's ability to carry out research initiatives, receive regulatory approvals or grants or experience positive effects or results from any research or study. Such forward-looking statements are estimates reflecting the Company's best judgment based upon current information and involve a number of risks and uncertainties, and there can be no assurance that the Company will actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements. As such, you should not place undue reliance on these forward-looking statements. Factors which could cause actual results to differ materially from those estimated by the Company include, but are not limited to, government regulation and regulatory approvals, managing and maintaining growth, the effect of adverse publicity, litigation, competition, scientific discovery, the patent application and approval process, potential adverse effects arising from the testing or use of products utilizing the DehydraTECH technology, the Company's ability to maintain existing collaborations and realize the benefits thereof, delays or cancellations of planned R&D that could occur related to pandemics or for other reasons, and other factors which may be identified from time to time in the Company's public announcements and periodic filings with the US Securities and Exchange Commission on EDGAR. The Company provides links to third-party websites only as a courtesy to readers and disclaims any responsibility for the thoroughness, accuracy or timeliness of information at third-party websites. There is no assurance that any of Lexaria's postulated uses, benefits, or advantages for the patented and patent-pending technology will in fact be realized in any manner or in any part. No statement herein has been evaluated by the Food and Drug Administration (FDA). Lexaria-associated products are not intended to diagnose, treat, cure or prevent any disease. Any forward-looking statements contained in this release speak only as of the date hereof, and the Company expressly disclaims any obligation to update any forward-looking statements or links to third-party websites contained herein, whether as a result of any new information, future events, changed circumstances or otherwise, except as otherwise required by law.
INVESTOR CONTACT:
George Jurcic - Head of Investor Relations
[email protected]
Phone: 250-765-6424, ext 202
SOURCE: Lexaria Bioscience Corp.




