Mercury Medical's Flow-Safe II+ Disposable BiLevel CPAP Device is Available for Acute Care Use and Surge Capacity Planning.
CLEARWATER, FL / ACCESSWIRE / August 2, 2021 / The FDA issued recent guidelines indicating that Bilevel and CPAP devices can be used to effectively help treat COVID-19 patients in Respiratory Distress potentially avoiding mechanical ventilation.
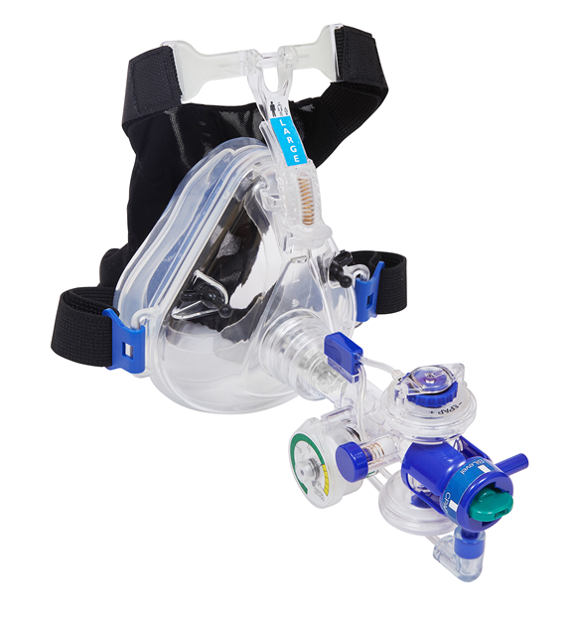
The patented Flow-Safe II+ is the first and only Disposable Bilevel CPAP ventilatory assist device available in the global market. This disposable Bilevel CPAP system includes a mask and manometer and optional filter that provides hospital and emergency clinicians with the components required to quickly set up the device and connect to an oxygen source for delivering verifiable Bilevel and CPAP therapies to patients in respiratory distress.
A recent article published in the American Journal of Emergency Medicine supports the use of the Flow-Safe product line concluding, "The Flow-Safe Disposable CPAP system can be as effective as NIMV in patients with Acute Cardiogenic Pulmonary Oedema (ACPO). Considering the overall improvement observed in the physiological blood gas and other parameters as well as the mortality and cost-related considerations, FSD-CPAP-S can be preferred in emergency services if there are insufficient NIMV devices."1
The disposable advantage reduces the need for costly capital equipment and is the clinical solution for situations where backup Bilevel / CPAP equipment is scarce or unavailable. Flow-Safe II+ has been used extensively in pre-hospital EMS environments and in acute care emergency rooms. The disposable feature has the added advantage of assisting in preventing potential cross contamination. These advantages make it an ideal solution when planning surge capacity for pandemics or natural disasters.
Flow-Safe II+ was introduced to the market in 2018 and has been awarded two prestigious industry awards, the 2018 EMS World Innovation Award and the 2019 JEMS Hot Products Award. This novel device was selected for both awards from over hundreds of submissions after a thorough review by panel of judges consisting of emergency medical services (EMS) product specialists, physicians, educators, managers and paramedics.
The United States Patent and Trademark Office (USPTO) has issued Flow-Safe II+ US Patent No.10,258,759 in 2019. In March 2020, the USPTO issued two new utility patents, US Patent No. 10,583,266 and 10,583,262 for the award-winning Flow-Safe II+ Disposable Bilevel CPAP device.
John Gargaro MD, President and CEO at Mercury Medical, states: "Mercury Medical believes that Flow-Safe II+ is a unique superior solution designed to quickly improve patients in respiratory distress with a cost-efficient device. The disposable feature assists in reducing hospital infection rates that are associated with reusable equipment, Mercury Medical has a rich experience in introducing innovative, clinically differentiated medical devices to market. We are extremely pleased to extend this device to the acute care market where there a need for Disposable Bilevel CPAP equipment."

About Mercury Medical
Mercury Medical is dedicated to delivering clinically differentiated critical care technology that saves lives throughout the world. For over 55 years clinicians have relied on Mercury Medical as their resource for bringing a legacy of innovative products to critical care areas of neonatal, anesthesia, respiratory and emergency markets in more than 58 countries. Mercury Medical's high-quality standards include ISO 13485 and ISO 9001 certifications.
For more information visit www.mercurymed.com
Contact:
D. Olson
Mercury Medical
727-573-4980
www.mercurymed.com
1. UZ, G.S. KIYAN, E. ÖZÇETE, et al., Is the flow-safe disposable continuous positive airway pressure (CPAP) system as effective as non-in..., American Journal of Emergency Medicine, https://doi.org/10.1016/j.ajem.2020.01.034
SOURCE: Mercury Medical Inc.




