Key Capital to advance development of breakthrough patented oral pill immunotherapeutics
NEW YORK, NY / ACCESSWIRE / November 24, 2020 / KEY CAPITAL CORPORATION (OTC PINK:KCPC) advises that Key Biotec, a division of Key Capital, has entered into agreement with Immunitor group interests ("Immunitor") to exclusively license its breakthrough and patented oral tableted platform vaccine and immunotherapeutic products for North, Central and South America (excluding Canada), the EU (excluding UK), Australia and New Zealand (the "Territory").
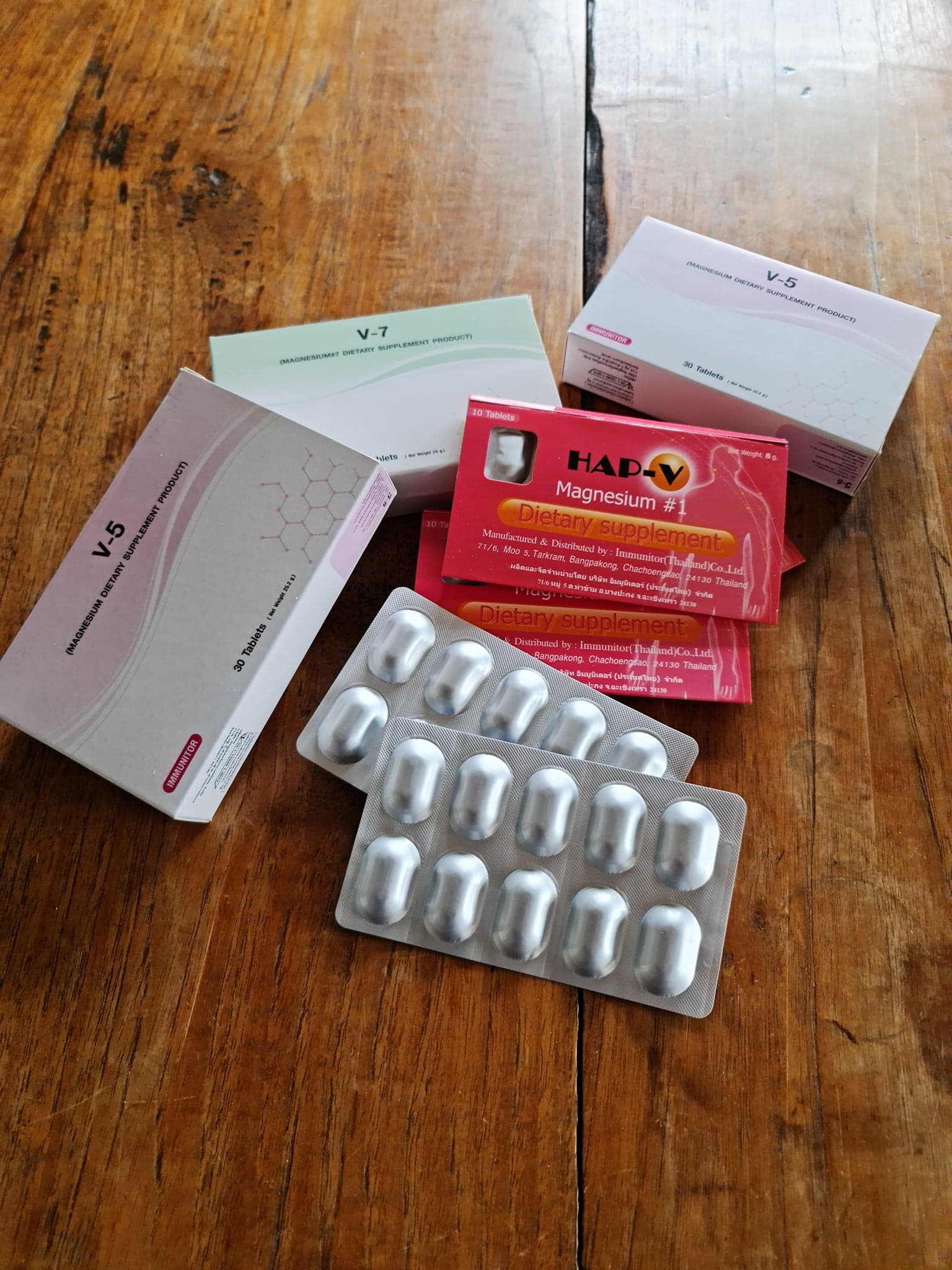
Immunitor is recognized as a pioneer in oral tableted immunotherapeutic vaccine science, having over the past 20 years developed 23 oral pill disease treatment products that are in various stages of clinical development. In each case, the patent-protected platform immunotherapeutics have consistently demonstrated success in diseases such as late stage cancers, tuberculosis, HIV/AIDS, hepatitis, and influenza, regularly offering better outcomes than current pharmaceutical options. This has been most significant, in many cases where current treatment options have failed or performed poorly.
Market: The major potential for the immunotherapeutics is in the prescription drug market, forecast to exceed US$1,500 billion by 2026, and in the vaccination market which is forecast to grow to US$104 billion by 2027.
Oral tableted immunotherapeutics: Immunitor immunotherapeutics primarily target the body's mucosal and innate immunity for broad and effective protection against invading pathogens and diseased cancer cells. This is achieved through the Immunitor proprietary technology platform enabling the active in oral tablets form to be protected from digestive degradation and thereby providing optimal access to the gut mucosa/microbiome which is responsible for the body's primary immune response functions.
Compelling use case: Immunitor immunotherapeutics treat disease and/or viral or bacterial infection through activation and optimization of the body immune system through its gut mucosa/microbiome. The oral pill platform technology overcomes the gut mucosa delivery challenges, promises superior immunotherapeutic performance, offers improved safety in having no side effects or toxicity, and avoids undesirable immune effects such as cytokine storm and autoimmune conditions. The patented oral tableting technology also provides major development and consumer cost benefits.
The many peer-reviewed publications of Immunitor clinical studies support the safety, performance, and enhanced efficacy of the Immunitor products comparative to current alternatives, which provide for the underlying use and commercialization potential of the immunotherapeutics to be quite dramatic.
Development history of Immunitor immunotherapeutics: The Immunitor journey for oral tableted immunotherapeutics development, pioneered by Dr. Aldar Bourinbaiar and Vichai Jirathitikal, commenced 20 years ago with an oral immunotherapeutic vaccine for HIV/AIDS. Since then Immunitor has progressively refined its technology and successfully trialed and patent-protected its products.
The clinical studies, funded through angel investors, were conducted largely in the Ukraine and Mongolia, leveraging major research cost advantages. The research has additionally been supported by three grants jointly from the Canadian government and the Bill and Melinda Gates Foundation, and also grants from the US State Department's Science & Technology Entrepreneurship Program award, the Ukraine Ministry of Science, and several through private foundations/interests.
A core attribute of Immunitor's patented platform technology is that almost any immunotherapeutic product can be manufactured and ready for clinical trials within weeks, as the Immunitor platform technology allows for a safe inactivated whole or part pathogen, virus, or other agent to be readily incorporated within the patent protected tableting process of the Immunitor platform technology.
Current regulatory situation: Several hundred thousand people to date have used Immunitor oral tableted immunotherapeutics in limited countries where some Immunitor products are approved for sale, and their use further confirming safety and efficacy. The Western and larger markets will require further and more extensive formal studies to secure the FDA (USA), EMA (EU), or TGA (Australia) registrations needed to allow marketing in these regions.
USA FDA: In the USA, Immunitor does not have any regulatory approvals for any of its products, however three Immunitor oral pill immunotherapeutics, Tubimod and Mycobacterium vaccae, each for treatment of TB, and Hepko-V5 for treatment of Hepatocellular carcinoma have current US FDA Orphan Drug Designation; and 16 clinical study trials for various indications, including many for cancers, are listed on https://clinicaltrials.gov/ct2/results?term=immunitor.
Advantages of Immunitor immunotherapeutics include: Development timelines, product cost, and time to market can be dramatically reduced, with further benefits including: Improved safety as products are not chemical compounds; Potential for better outcomes; Cost is a fraction of most other treatments and therefore affordable to larger populations; Oral pills are room temperature stable and do not require cold-chain custody, transportation or storage; Pill manufacturing can easily scale up to multi-millions of doses per day; Needle-free and not requiring clinical administration; Overcomes needle phobia; Self-administration can dramatically enhance scalability.
Key Biotec licensing and partnering: Key Capital Corporation Chairman, Peter Boonen stated: "Immunitor's patented platform technology and their 20-year successful development of the science and product candidates to date, provides a solid business case and an extraordinary opportunity for the Company and Key Biotec. We look forward to supporting Immunitor in progression of its three FDA Orphan Drug Designated candidates, and in further development of its oral tablet immunotherapeutics portfolio products throughout our licensed Territory, and for our mutual benefit in accordance with our License Agreement partnering."
Jonathan Wong, Lead Scientific Advisor and Immunitor consultant who formerly headed development of novel antimicrobial drugs against intracellular pathogens including pandemic viruses for the Canadian government at DRDC Suffield added: "The potential for progressing a class of oral pill immunotherapeutic disease treatment products that are safe and effective is exciting, however of more importance is getting these products approved and to market."
COVID-19, viruses, and infectious disease: Infectious diseases represent a major threat to human lives as seen by the rapid pandemic spread of COVID-19 to over 58 million infections and some 1.4 million deaths to date. There is no doubt the world will regularly be subject to further future pandemic threats. In this regard a key attribute of the Immunitor Immunotherapeutic platform is that, being oral pill therapeutic vaccines that are not pharmaceutical compounds, synthetic or genetically engineered, they can be readily formulated, trialed, and subject to approvals, easily and cost-effectively mass distributed.
A novel and groundbreaking Immunitor oral tableted immunotherapeutic vaccine is currently being studied for SARS-CoV-2 /COVID-19 in several Asian regions. Significant potential advantages of this oral pill immunotherapeutic vaccine, subject to continued development success, are its safety, its highly advantageous time-to-market and cost benefits, its mass-market distribution scalability, and that it is therapeutic as well as most likely prophylactic.
Key Capital Corporation: The Company Chairman is currently leading a Company reorganization focused on securing dynamic proven corporate and pharma industry management. The Company's immediate operational objective will be the further development of the oral pill immunotherapeutics opportunity within its Territory in association with its Immunitor partnering, and particularly on advancing the Orphan Drug Designated cancer candidate, along with early focus on disease conditions with unmet needs.
Regarding the current trading situation of the Company's securities, the Company will be working with its advisors to ensure reinstatement of trading is resumed within the next six months.
For further information see: https://keybiotec.com and https://keycapitalcorp.com
For all inquiries please contact:
Key Capital at: +1 (646) 401-0177, or Peter Boonen, Chairman at: [email protected]
Disclaimer: Statements made in this press release that express the Company or management's intentions, plans, beliefs, expectations, or predictions of future events, are forward-looking statements. The words "believe," "expect," "intend," "estimate," "anticipate," "will" and similar expressions are intended to further identify such forward-looking statements, although not all forward-looking statements contain these identifying words. Those statements are based on many assumptions and are subject to many known and unknown risks, uncertainties and other factors that could cause the Company's actual activities, results or performance to differ materially from those anticipated or projected in such forward-looking statements. The Company cannot guarantee future financial results, levels of activity, performance or achievements and investors should not place undue reliance on the Company's forward-looking statements.
SOURCE: Key Capital Corporation




