COMPANY'S EMERGENCY USE (EUA) APPLICATION TO BE UPDATED IN THE NEXT WEEK AS MANUFACTURING SPECIFICATIONS FINALIZED, DISCUSSION OF GENVIRO! UNIQUE QUALITIES ENSUES
LOS ANGELES, CA / ACCESSWIRE / March 23, 2020 / Decision Diagnostics Corp. (OTC PINK:DECN) is an 18-year old, diabetes-focused bio-technology development firm, manufacturer, quality plan administrator, FDA registered medical device customer support organization, and exclusive worldwide sales and regulatory process agent for the GenUltimate! ("Sunshine") diabetes test strip, its GenAccord! Enhance ("Caterpillar") strip and meter systems for the uninsured and under-insured, its GenChoice! ("Ladybug") test strip now in the later stages of FDA 510(k) prosecution, and its GenUltimate! Precis products manufactured for International markets, and its GenViro! Rapid Kits for the real time testing for the Coronavirus (COVID-19) now seeking FDA Emergency Waiver (EUA).
Today, DECN announces that the company, through its advanced development team in Korea, have finalized the configuration of the GenViro! Swift Kit test strip that will go into production just as soon as the FDA grants emergency status to the DECN product. The company's methodology employs a simple, easy to use, swift (15 seconds and faster than Rapid) method for determining the presence of a virus in blood lyced into blood plasma. Blood sample requirements are 1-2 microliters. The kits will be manufactured for DECN in Korea by its contract manufacturer, the same company that manufactures our diabetic test strips. While the manufacturing will occur in Korea, the test strip is 100% of American design.

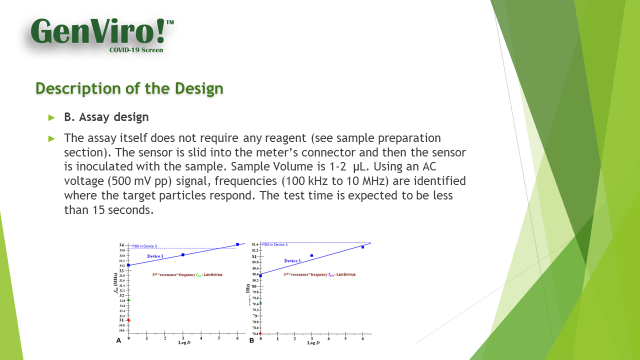
Keith Berman, CEO of DECN commented, "The news channels are filled with talk about the coming rapid test kits for the detection of the virus Covid-19. These kits are recent creations based on traditional chemistry methods such as anti-body/antigen analysis or PCR (the nose swab RNA method). While these methods offer a path to results, we have not found any that will give a patient a result in less than minutes (and we have our doubts), or are methods that can provide on the spot analysis.
Further these methods and variations of same, typically require analyzers that are in the instrument arsenals of large hospitals or large commercial laboratories, not the shopping center parking lot or the flu shot clinic at the local pharmacy, or the meeting hall at a church. GenViro! provides results in 15 seconds, based on a small finger prick blood sample. The method is safe, effective, and its biggest benefit to the healthcare system is that the device can be used to screen out the 97% or 98% of those tested that are negative for COVID-19. Our method is quicker, provides the desired result, is much cheaper, and effective."
Serology Testing for COVID-19
According to the Johns Hopkins School of Medicine, "Serology tests are blood-based tests that can be used to identify whether people have been exposed to a particular pathogen. Serology-based tests analyze the serum component of whole blood. The serum includes antibodies to specific components of pathogens, called antigens. These antigens are recognized by the immune system as foreign and are targeted by the immune response. These types of tests are often used in viral infections to see if the patient has an immune response to a pathogen of interest, such as influenza, or in this case COVID-19. These methodologies are a focus of current test kit methodologies, particularly what are called rapid kits, which are not very rapid, and not self-contained so therefore only a partial kit. These tests have been around at least since the 1980s. GenViro! is not a Serology methodology.
PCR Based Testing for Coronavirus ("Take a Nose Swab")
PCR based (RNA) testing of virus and infections has been around since the early 1980s and make up a large number of methods used to detect Covid-19. For example the much discussed Roche methodology detects the genetic signature (RNA) of the SARS-CoV-2 virus in swab samples that a healthcare provider collects from the back of the patient's throat or nose. Most alternative methods work in the same manner. Test turn-around time, usually is 4 hours. However, the testing does not occur at the location where the nose swab is taken. Also, instrumentation needed to complete the tests and report them are expensive. Many feel that In the United States, the slow rollout of coronavirus PCR (nose swab) tests has been widely attributed to a combination of previously stringent FDA rules, and the slower, older methodologies. The DECN GenViro! kit does not employ a nose swab, rather it receives its small blood sample through a finger prick, is self-contained and disposable, does not require a hospital or clinical lab based instrument for analysis, and it only takes 15 seconds, a minute fraction of the nose swab tests.
Mr. Berman concluded, "DECN is also creating what we call our affirmation test, a kit that will affirm a positive reading from the GenViro! Screener. Similar in nature to the GenViro! screener, and readable by the Avantage meter, the affirmation test will be serology based, but will use our unique and charmed electrochemical impedance spectroscopy. We anticipate being tens of millions of GenViro! kits down the road when our serology version of Genviro! is completed. These are the most exciting days at DECN."
ABOUT DECISION DIAGNOSTICS CORP
Decision Diagnostics Corp. is the leading manufacturer and worldwide distributor of diabetic test strips engineered to operate on legacy glucose meters. DECN's products are designed to operate efficiently and less expensively on certain glucose meters already in use by almost 7.5 million diabetics worldwide. With new inspired technology diabetic test strips already in the final stages of development, DECN products compete on a worldwide scale with legacy manufacturers currently selling to 71+ percent of a $15+ billion at-home testing market. The company's new GenViro!TM product designed to test for the Coronavirus Covid-19, is not yet available in the United States or Puerto Rico but Emergency Waivers are in process, and the product concept has been presented to officials in Washington, DC by invitation.
Forward-Looking Statements:
This release contains the company's forward-looking statements which are based on management's current expectations and assumptions as of March 20, 2020, regarding the company's business and performance, its prospects, current factors, the economy, and other future conditions and forecasts of future events, circumstances, and results.
CONTACT INFORMATION:
Decision Diagnostics Corp.
Keith Berman (805) 446-2973
[email protected]
www.genultimate.com
www.genultimatetbg.com
www.pharmatechdirect.com
SOURCE: Decision Diagnostics Corp.




