Company posts initial forecast for its new GenAccord!, moves two new products GenUltimate! precis TBG and GenCambre! into advanced development, readies patents for GenUltimate TBG & GenExpidient!
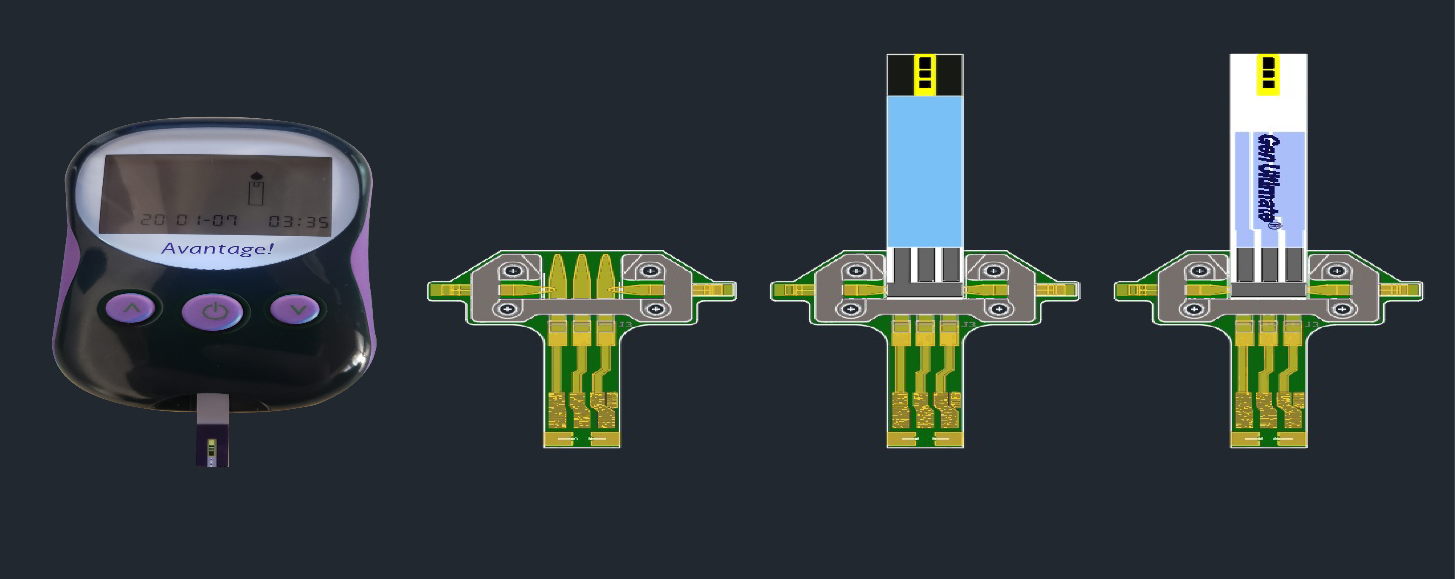
LOS ANGELES, CA / ACCESSWIRE / February 20, 2020 / Decision Diagnostics Corp. (OTC PINK:DECN) is an 18-year old, diabetes-focused bio-technology development firm, manufacturer, quality plan administrator, FDA registered medical device support organization, and exclusive worldwide sales and regulatory process agent for the GenUltimate! ("Sunshine") diabetes test strip, its GenAccord! systems for the uninsured and under-insured, its GenChoice! ("Ladybug") test strip now in the later stages of FDA 510(k) prosecution, its mate! GenUltimate Precis products manufactured for Eastern European International markets. The company also markets its PetSure! test strip for the diabetic testing of dogs and cats, a diagnostic specifically designed to run on the market leading Zoetis Alpha Trak meter system as well as the GenUltimate! 4Pets Test strip and Avantage! meter. Finally the company has certified as market ready its GenUltimate! TBG ("Dragonfly") diabetes testing system, and announces the completion of its Universal Translator device GenExpidient!, a device that serves as a universal translation device and dramatically speeds development of alternative test strips that will run on legacy systems.
The company also announced that with the completion of development of the GenExpiedient! Universal Translator, two products in development have been moved to Advanced Development (approx. 120 days to commercial viability), GenCambre! and the TBG version of GenUltimate! Precis TBG. Precis TBG will sell through our Russian Federation partner.
Keith Berman, CEO of Decision Diagnostics stated "Despite our limited resources, or perhaps because we have fewer resources, we have been able to channel our activities into products that can be introduced to the market quickly. In 2020 we will have introduced GenUltimate! TBG, GenAccord!, GenCambre! and now GenExpidient! "


Mr. Berman continued, "We have great hopes for GenExpidient! This device that will allow similar commercial systems with different glucometers and/or meters of different vintage to work with the same test strip of our development. This has brought another 6 legacy products into the realm of our brand. GenExpidient! is eminently patentable, and along with our GenUltimate TBG are the subject of three patent applications now underway."
Mr. Berman concluded, "We have posted our "cocktail napkin" forecast for our GenAccord! product line. We were a little late with this forecast, primarily because we have been granted additional territories and had to amend the forecast. The forecast will again be revised to take into account our new retail partner who has agreed to carry the GenAccord! line in over 2000 retail stores. One thing that can be said about this partner is that their clientel are fanatical about the brand name products sold at the 2000+ retail stores. Back to the forecast that is posted: for the most part, the forecast breaks down and displays by country. This forecast can be viewed at: http://decisiondiagnostics.co/docs/new/Exhibit%20D.pdf
A password to the file will be required. Shareholders and interested parties who wish to view the forecast can call the company and after being asked to answer a few questions, will be provided the password. The password is not "password." The forecast is viewable on Microsoft, Google, and Firefox browsers.
ABOUT DECISION DIAGNOSTICS CORP
Decision Diagnostics Corp. is the leading manufacturer and worldwide distributor of diabetic test strips engineered to operate on legacy glucose meters. DECN's products are designed to operate efficiently and less expensively on certain glucose meters already in use by almost 7.5 million diabetics worldwide. With new inspired technology diabetic test strips already in the final stages of development, DECN products compete on a worldwide scale with legacy manufacturers currently selling to 71+ percent of a $15+ billion at-home testing market. The company's GenUltimate TBG product is not yet available for sale in the United States or Puerto Rico but is on sale Internationally. Our GenAccord! product has been launched Internationally.
Forward-Looking Statements:
This release contains the company's forward-looking statements which are based on management's current expectations and assumptions as of February 19, 2020 regarding the company's business and performance, its prospects, current factors, the economy, and other future conditions and forecasts of future events, circumstances, and results.
CONTACT INFORMATION:
Decision Diagnostics Corp.
Keith Berman (805) 446-2973
[email protected]
www.genultimate.com
www.genultimatetbg.com
www.petsureteststrips.com
www.pharmatechdirect.com
SOURCE: Decision Diagnostics Corp.




