GOLDEN, CO / ACCESSWIRE / June 18, 2019 / Vitro Diagnostics, Inc. (OTCQB: VODG), dba Vitro Biopharma, announced its 2nd quarter ended April 30th 2019 financial results of operations.
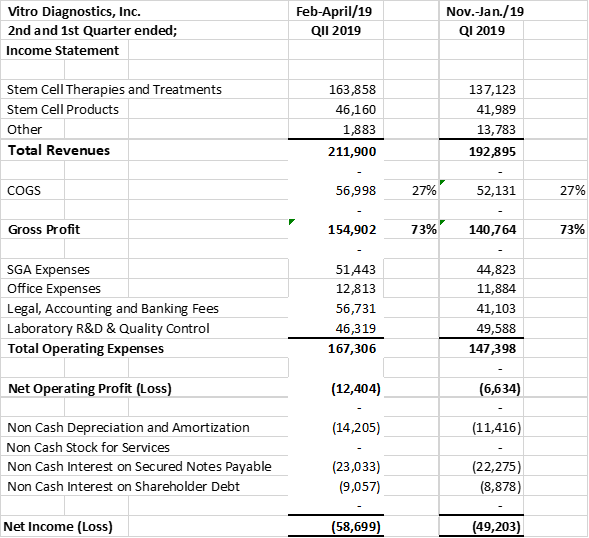
Vitro Diagnostics Inc. ("Vitro Biopharma") is pleased to announce a record 2nd quarter in Stem Cell Revenues. Vitro Biopharma recorded 2nd quarter revenues of $211,900 vs $125,103 an increase of 69% over the same comparative quarter last year. In addition, Stem Cell treatments accounted for 77% of the revenues up from 53% of the revenues in the prior comparative quarter last year. Current quarter stem cell revenues were $163,858 for the 2nd quarter ended April 30th, 2019 vs $66,537 for the second quarter ended April 30th, 2018. In the six months ended April 30, 2019, revenues were 90% higher than the comparable period in 2018, $404,794 in 2019 verses $213,071 in 2018.
The company's gross profit margins maintained at approximately 73% for the current quarter, prior quarter, six months ended quarter and prior comparative quarter.
Overall operating expenses increased in the quarter by $46,226 to $167,307 from $121,081 in the prior year's comparative quarter. The increase in expenses reflects the increased costs of sales and marketing, quality certifications (ISO 9001, ISO 13485, CLIA) accounting, regulatory, product and business development. This represents the company's investment in its executive team to support increased business development activities in the Cayman Islands, Bahamas and it's USA and international Cosmetic partnership with Limitless MD, led by Jack Zamora, MD.
During the quarter the company achieved and pursed the following objectives:
- Began expansion of its manufacturing capacity
The company is doubling its laboratory and manufacturing facilities and expanding its clean room by 100% in size and capacity. This new facility is expected to be online during the 1st quarter of next year. The company signed an expanded equipment lease facility with Thermo Fisher Financial for over $100,000 to invest in an automated filling machine, advanced cell counting device and additional incubators to process its AlloRx Stem Cells™ and its Limitless MD™ Serum product. The expansion is split into two phases; the current phase adds 2 new incubators; one immediately and another within 6 months providing Vitro Biopharma with the capacity to process 24 Billion AlloRx cells a month. This represents approximately $ 6 Million of AlloRx Stem Cell™ Vitro Biopharma revenue capacity per year. Furthermore, the completion of the 2nd clean room processing facility at the beginning of the 2020 year will expand our capacity to approximately 100 Billion AlloRx Stem Cells™ a month or approximately $1.7 Million of AlloRx Stem Cell™ Vitro Biopharma revenue capacity per month. This would give Vitro Biopharma a revenue run rate capacity of $20M a year.
Our increased capacity is rigorously controlled by our Quality Management System, now certified to the ISO9001 Quality Standard and the ISO13485 Medical Device Standard as well. This provides GMP-compliant manufacturing of the highest quality stem cells/medical devices for clinical trial testing to provide further evidence of safety and efficacy for treatment for a wide variety of indications. Highly regulated GMP biologics manufacturing within an FDA-compliant facility provides numerous opportunities to the Company to drive strong revenue growth. We are presently focused on our partnerships in the Caribbean with DVC Stem in Grand Cayman Island, Limitless MD in the US and emerging opportunities in the Commonwealth of the Bahamas. We are actively pursuing other partnership opportunities as well.
- NutraVivo™ manufacturing update:
We had previously finalized an agreement with an a CMO to reformulate and produce our NutraVivo™ brand of nutraceuticals. NutraVivo™ contains natural substances that activate the body's own stem cells to enhance recovery from injury such as TBI, stroke, MS, PD and other autoimmune and neurological diseases. The revised NutraVivo™ product will be offered as a private label product to Limitless MD clinics and is being implemented as supplemental support to clinical treatments now ongoing in the Cayman Islands. Patients report positive benefits from NutraVivo™ therapy following stem cell transplants including increased overall energy and enhancement of improved motor function in MS patients. We are currently testing the new formulation for its commercial release and implementing a name change to STEMulize™ to reflect its use as a nutraceutical stem cell activator.
- Expanded Revenue from Limitless MD™ Serum
The Company's cosmetic stem cell serum private labelled as Limitless MD Serum is being applied as a topical cosmetic beautification product that is used in conjunction with various skin resurfacing devices. Limitless MD™ continues to expand the its base of authorized partner clinics and revenues grew 30% in the quarter to approximately $130,000 vs $100,000 in the prior quarter. This also compares to $130,000 in the current quarter of 2019 vs $6,000 in the prior comparative quarter of 2018. These developments have been in process for the last year and the Joint Development and Supply Agreement dated May 15th 2018 between Vitro Biopharma and Jack Zamora is now producing material results. The agreement calls for minimum orders to remain exclusive. The agreement reaches a minimum level of performance of $1,000,000 annualized by the six-month ended period of June 30th 2020.
Limitless MD™ Serum is revolutionizing cosmetic care. The results are delivering reduced down time and an improved beautification experience. This is one of the fastest growing revenue streams for Vitro Biopharma. The Limitless MD product offers many benefits including increased beautification, hydration and improved results compared to alternative products. We work with a variety of regulatory experts to assist us in the appropriate regulatory pathway. At this point it is regulated as a cosmetic device but may be reclassified based on regulatory advice and guidance.
www.jackzamoramd.com www.limitlessmdcell.com
Limitless MD™ also has an exclusive agreement to distribute AlloRx Stem Cells™ into the countries of Saudi Arabia, U.A.E., and Columbia. The first trial run clinic in Dubai is being brought on board in the third quarter of 2019. The agreement calls for minimum commitments to maintain exclusivity and provides for minimum revenue of $250,000 annually in 2020.
Vitro Biopharma's OEM cosmetic topical serum is being distributed by Limitless MD™ into cosmetic clinics that are providing the topical treatment as a beautification product used in conjunction with various skin resurfacing devices. To date the company's product is being offered in 7 cosmetic clinics.
- New Clinical Trial of Musculoskeletal Conditions in the Bahamas
Subsequent to its 2nd quarter, the Company received Provisional approval from the National Stem Cell Ethics Committee (NSCEC) of the Ministry of Health of the Commonwealth of the Bahamas for a clinical trial entitled ''Vitro Biopharma Allogeneic Mesenchymal Stem Cell Therapy for Musculoskeletal Conditions'':
This broadens Vitro Biopharma's expansion into highly regulated stem cell trials in collaboration with the Nassau-based Medical Pavilion of the Bahamas (TMPB). http://www.tmp-bahamas.com.
We will now be able to extend stem cell therapy based on our novel, patent-pending AlloRx Stem Cell™ product to a variety of musculoskeletal conditions. These include OA of any joint, ACL/MCL tear, Achilles tendon rupture, rotator cuff injury, tennis elbow and herniated disc that are highly prevalent and have few disease-modifying options. It is important to note that many stem cell treatments now performed are problematic due to limited potency and failure to meet basic criteria of stem cells. Also, contamination due to poor production methods that are not in compliance with FDA regulations can cause serious complications. Vitro Biopharma has operated a highly regulated, FDA-compliant commercial biologics manufacturing operation for several years and is cGMP compliant, ISO 9001Certified, ISO 13485 Certified, CLIA Certified and FDA registered. All manufacturing occurs in a certified sterile clean room with extensive and advanced testing to assure the absence of contamination. Furthermore, in numerous patients treated to date by IV infusion of AlloRx Stem Cells™ there have been no significant adverse events.''
The company is partnered with Dr. Conville Brown, MD, MBBS, FACC, FESC, PhD, the founder and CEO of the Medical Pavilion of the Bahamas who is the Principal Investigator of this trial and director of its clinical administration. Dr Brown was instrumental in the establishment of the NSCEC in the Bahamas.
About the Medical Pavilion of the Bahamas: TMPB operates within a 40,000 square foot building as a partnered care specialty medical facility with 10 different centers in various areas including cardiology, cancer, clinical research and kidney disease. One of the centers is the Partners Stem Cell Centre, where the present trial will be conducted. The Partners Stem Cell Centre provides an environment to conduct stem cell research and clinical trials under the model of ''FDA rigor in a Non-FDA Jurisdiction'' TMPB employs 20 medical specialists in various fields. See www.tmp-bahamas.com for additional information.
The company expects to begin patient enrollment for the clinical trial in late QIV but does not expect revenue contribution until the end of the fiscal year.
- Expansion of revenues from the clinical trials in the Cayman Islands:
During the quarter Vitro Biopharma continued to expand its clinical trial business in the Cayman Islands with its partner www.DVCStem.com under the joint IRB covering inflammatory conditions. Treatments to date have covered MS (Multiple Sclerosis), OA(Osteo Arthritis),PD (Parkinson's) and other inflammatory diseases. DVC Stem specializes in clinical stem cell regenerative medicine utilizing Vitro Biopharma's AlloRx Stem Cells™ under approval of the Ministry of Health of the Cayman Islands.
Our overall objectives are to support high quality offshore medical stem cell tourism with clinical trial partners worldwide.
- Expanded our Patent & Intellectual Property Portfolio
The Company has several patent applications (11) pending in the US and foreign jurisdictions. These patents cover our AlloRx Stem Cell™ line and various aspects of our NutraVivo™ stem cell activation products & processes as well as specific diagnostic tests of stem cell activity and therapeutic effectiveness. During the quarter, the Company has responded to office actions and continues to vigorously prosecute & expand its patent filings.
Dr. Jim Musick, CEO of Vitro Biopharma, said, "We are very pleased to report the results of operations through our 2nd fiscal quarter in 2019. We experienced strong revenue growth while our losses decreased at a faster rate. The company expects with continued revenue increase from all revenue categories that it will be cash flow positive in the next few quarters and report its first net profit in the 2020 time frame.
We have added several regulatory certifications and outside oversight of our bio manufacturing operations. We are now ISO9001, ISO13485 and CLIA certified and cGMP compliant. Our regulatory certifications allow us to gain offshore IRB approvals, e.g., in the Commonwealth of the Bahamas and other countries since the ISO Standards are internationally recognized. Certification to these rigorous standards are often required to perform manufacturing operations supporting IRB-approved clinical trials, especially in foreign jurisdictions.
Our stem cell products are distinctly superior to stem cell transplants in the USA. The latter usually involve use of impure products lacking validation as stem cells and containing insufficient numbers of stem cells to achieve therapeutic benefits. These are produced without regulatory oversight and have been known to cause serious adverse effects. Hence the use of highly purified and well characterized stem cells (AlloRx Stem Cells™) is needed to provide safety and efficacy in regenerative medicine therapies.
In summary, Vitro Biopharma is advancing as a key player in regenerative medicine with 10+ years experience in the development and commercialization of stem cell products for research, recognized by a Best in Practice Technology Innovation Leadership award for Stem Cell Tools and Technology and a growing track record of successful translation to therapy. We plan to leverage our proprietary technology platform to the establishment of international Stem Cell Centers of Excellence and regulatory approvals in the US and worldwide."
Sincerely yours,
James R. Musick, PhD.
President, CEO & Chairman of the Board
www.vitrobiopharma.com
Forward-Looking Statements
Statements herein regarding financial performance have not yet been reported to the SEC nor reviewed by the Company's auditors. Certain statements contained herein and subsequent statements made by and on behalf of the Company, whether oral or written may contain "forward-looking statements". Such forward looking statements are identified by words such as "intends," "anticipates," "believes," "expects" and "hopes" and include, without limitation, statements regarding the Company's plan of business operations, product research and development activities, potential contractual arrangements, receipt of working capital, anticipated revenues and related expenditures. Factors that could cause actual results to differ materially include, among others, acceptability of the Company's products in the market place, general economic conditions, receipt of additional working capital, the overall state of the biotechnology industry and other factors set forth in the Company's filings with the Securities and Exchange Commission. Most of these factors are outside the control of the Company. Investors are cautioned not to put undue reliance on forward-looking statements. Except as otherwise required by applicable securities statutes or regulations, the Company disclaims any intent or obligation to update publicly these forward-looking statements, whether as a result of new information, future events or otherwise.
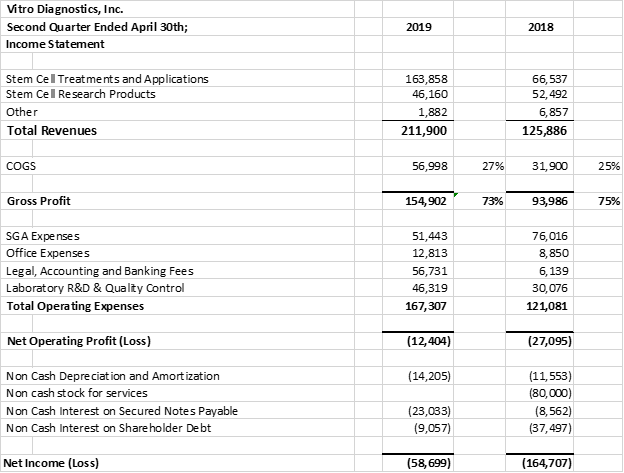
The company provides its financial information for investor purposes only, the results published are not audited or necessarily SEC or GAAP compliant.
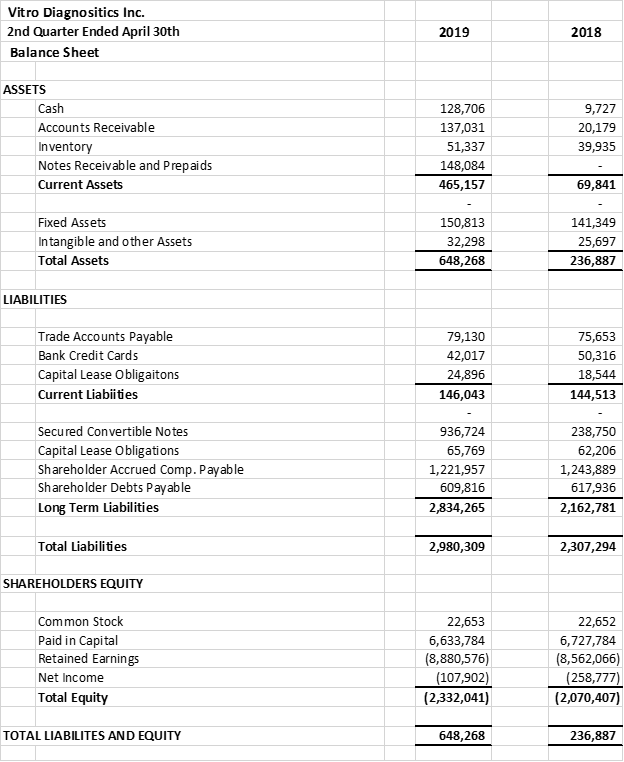
The company provides its financial information for investor purposes only, the results published are not audited or necessarily SEC or GAAP compliant
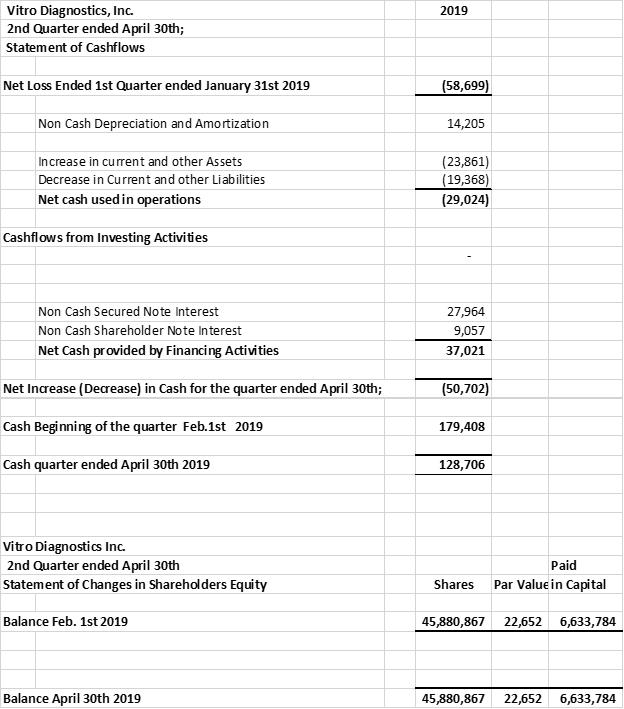

The company provides its financial information for investor purposes only, the results published are not audited or necessarily SEC or GAAP compliant.
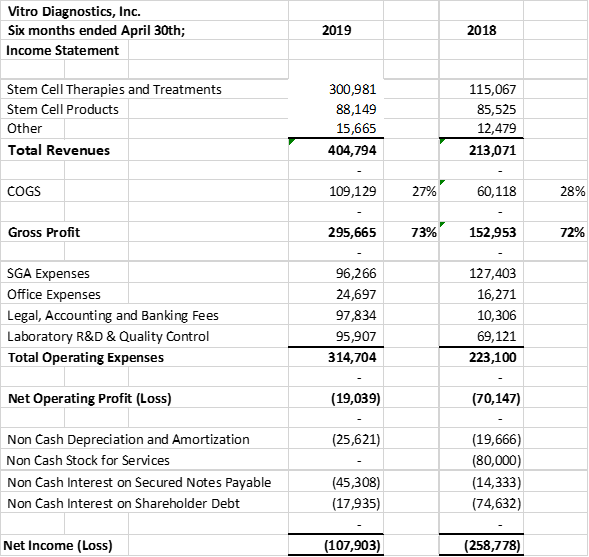
The company provides its financial information for investor purposes only, the results published are not audited or necessarily SEC or GAAP compliant.
The company provides its financial information for investor purposes only, the results published are not audited or necessarily SEC or GAAP compliant.
CONTACT:
Dr. James MusickChief Executive Officer
Vitro BioPharma
(303) 999-2130 Ext. 3
E-mail: [email protected]
www.vitrobiopharma.com
SOURCE: Vitro Diagnostics, Inc.




