TORRANCE, CA / ACCESSWIRE / December 14, 2018 / Asclemed USA Inc is voluntarily recalling 20 lots of Dyural-40 and 61 lots of Dyural-80, to the user level. The products include recalled Sodium Chloride, USP, 0.9% manufactured by Fresenius Kabi, which has been recalled due to product labeling incorrectly stating stoppers do not contain latex.
For the population most at risk, those with a severe allergic reaction to latex, there is probability of an anaphylactic reaction, and this could result in hospitalization or death. To date, Asclemed USA Inc has not received any reports of adverse events related to this recall.
The products are Dyural-40 convenience kits packaged in plastic trays and Dyural-80 convenience kits packaged in plastic trays, containing Sodium Chloride, USP, 0.9% by Fresenius Kabi.
The affected Dyural-40 lots include the following:
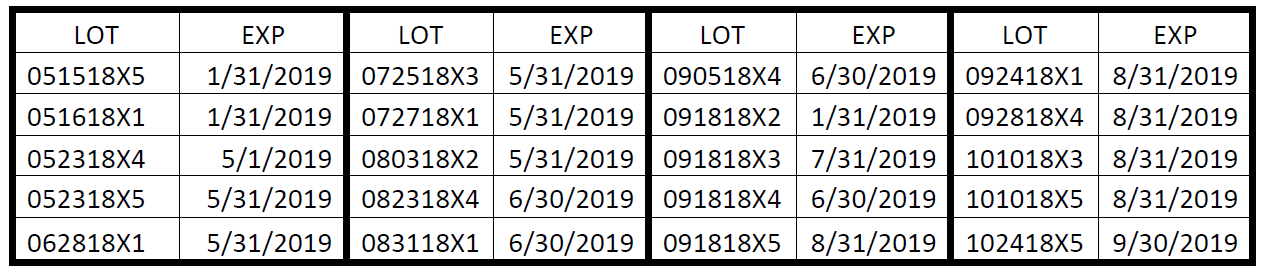
The affected Dyural-80 lots include the following:
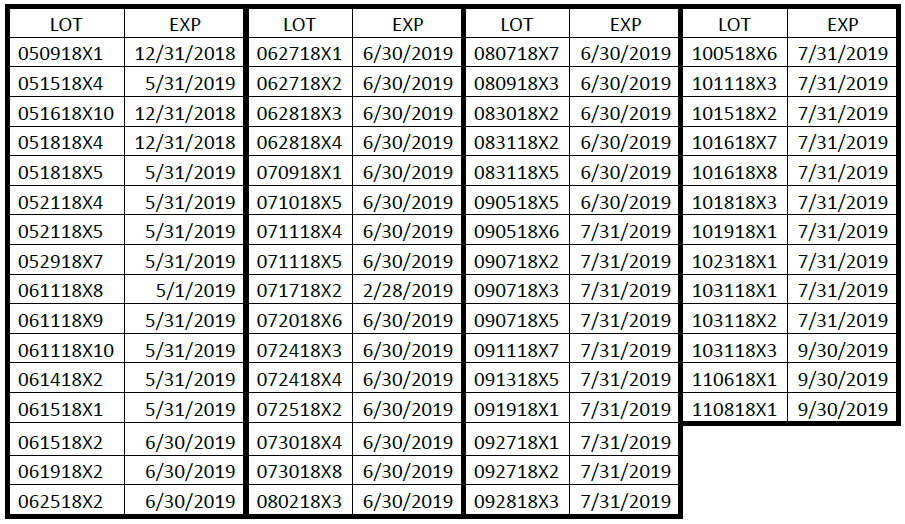
The products can be identified by lot and expiration stamped on the front of each convenience kit. Product was distributed Nationwide to distributors and physicians.


Asclemed USA Inc is notifying its distributors and customers by email and is arranging for return of all recalled products. Distributors and physicians that have Dyural-40 or Dyural-80 which is being recalled should stop using them and return to place of purchase.
Consumers with questions regarding this recall can contact Asclemed USA Inc by calling (310) 320-0100 ext. 120 Monday through Friday from 7:30am to 4:00pm PST or emailing [email protected]. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm1
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm2 or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Company Contact:
Christina Hopson
(310) 320-0100 ext. 120
SOURCE: Enovachem Pharmaceuticals




